Summary
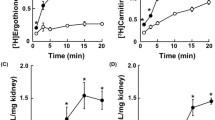
Rabbit kidney proximal convoluted tubule (RPCT) and proximal straight tubule (RPST) cells were independently isolated and cultured. The kinetics of the sodium-dependent glucose transport was characterized by determining the uptake of the glucose analog alpha-methylglucopyranoside. Cell culture and assay conditions used in these experiments were based on previous experiments conducted on the renal cell line derived from the whole kidney of the Yorkshire pig (LLC-PK1). Results indicated the presence of two distinct sodium-dependent glucose transporters in rabbit renal cells: a relatively high-capacity, low-affinity transporter (Vmax=2.28±0.099 nmoles/mg protein min, Km=4.1±0.27 mM) in RPCT cells and a low-capacity, high-affinity transporter (Vmax=0.45±0.076 nmoles/mg protein min, Km=1.7±0.43 mM) in RPST cells. A relatively high-capacity, low-affinity transporter (Vmax=1.68±0.215 nmoles/mg protein min, Km=4.9±0.23 mM) was characterized in LLC-PK1 cells. Phlorizin inhibited the uptake of alpha-methylglucopyranoside in proximal convoluted, proximal straight, and LLC-PK1 cells by 90, 50, and 90%, respectively. Sodium-dependent glucose transport in all three cell types was specific for hexoses. These data are consistent with the kinetic heterogeneity of sodium-dependent glucose transport in the S1–S2 and S3 segments of the mammalian renal proximal tubule. The RPCT-RPST cultured cell model is novel, and this is the first report of sodium-dependent glucose transport characterization in primary cultures of proximal straight tubule cells. Our results support the use of cultured monolayers of RPCT and RPST cells as a model system to evaluate segment-specific differences in these renal cell types.
Similar content being viewed by others
References
Alavi, N.; Spangler, R. A.; Jung, C. Y. Sodium-dependent glucose transport by cultured proximal tubule cells. Biochim. Biophys. Acta 899:9–16; 1987.
Barfuss, D. W.; Schafer, J. A. Differences in active and passive glucose transport along the proximal nephron. Am. J. Physiol. (Renal Fluid Electrolyte Physiol. 10) 240:F322-F332; 1981.
Bell, C. L.; Tenenhouse, H. S.; Seriver, C. R. Initiation and characterization of primary mouse kidney epithelial cultures. In Vitro Cell. Dev. Biol. 24:683–695; 1988.
Berry, C. A. Heterogeneity of tubular transport processes in the nephron. Annu. Rev. Physiol. 44:181–201; 1982.
Blais, A.; Morvan-Baleynaud, J.; Friedlander, G.; Grimellec, C. L. Primary culture of rabbit proximal tubules as a cellular model to study nephrotoxicity of xenobiotics. Kidney Int. 44:13–18; 1993.
Boogaard, P. J.; Mulder, G. J.; Nagelkerke, J. F. Isolated proximal tubular cells from rat kidney as an in vitro model for studies on nephrotoxicity. I. An improved method for preparation of proximal tubular cells and their functional characterization by alpha-methylglucose uptake. Toxicol. Appl. Pharmacol. 101:135–143; 1989.
Boogaard, P. J.; Zoeteweij, J. P.; vanBerkel, T. J. C.; van'tNordende, J. M.; Mulder, G. J.; Nagelkerke, J. F. Primary culture of proximal tubular cells from normal rat kidney as an in vitro model to study mechanisms of nephrotoxicity. Toxicity of nephrotoxicants at low concentrations during prolonged exposure. Biochem. Pharmacol. 39:1335–1345; 1990.
Chesney, R.; Sacktor, B.; Kleinzeller, A. The binding of phloridizin to the isolated luminal membrane of the renal proximal tubule. Biochim. Biophys. Acta 332:263–277; 1974.
Chung, S. D.; Alavi, N.; Livingston, D.; Hiller, S.; Taub, M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J. Cell Biol. 95:118–126; 1982.
Clancey, C. J.; Lever, J. E. Differential regulation of three glucose transporter genes in a renal epithelial cell line. J. Cell. Physiol. 185:244–252; 2000.
Cornish-Bowden, A. Analysis of enzyme kinetic data. Oxford: Oxford University Press; 1995.
Courjault-Gautier, F.; Chevalier, J.; Abbou, C. C.; Chopin, D. K.; Toutain, H. J. Consecutive use of hormonally defined serum-free media to establish highly differentiated human renal proximal tubule cells in primary culture. J. Am. Soc. Nephrol. 5:1949–1963; 1995.
Elfarra, A. A.; Anders, M. W. Renal processing of glutathione conjugates. Role in nephrotoxicity. Biochem. Pharmacol. 33:3729–3732; 1984.
Garvin, J. L. Glucose absorption by isolated perfused rat proximal straight tubules. Am. J. Physiol. (Renal Fluid Electrolyte Physiol. 28) 259:F580-F586; 1990.
Hediger, M. A.; Budarf, M. L.; Emanuel, B. S.; Mohandas, T. K.; Wright, E. M. Assignment of the human intestinal Na+/glucose cotransporter gene (SGLT1) to the q11.2-qter region of chromosome 22. Genomics 4:297–300; 1989.
Hirayama, B. A.; Lostao, M. P.; Panayotova-Heiermann, M.; Loo, D. D. F.; Turk, E.; Wright, E. M. Kinetic and specificity differences between rat, human and rabbit Na+-glucose cotransporters (SGLT1). Am. J. Physiol. (Gastrointest. Liver Physiol. 33) 270:G919-G926; 1996.
Horio, M.; Fukuhara, Y.; Orita, Y.; Nakanishi, T.; Nakahama, H.; Moriyama, T.; Kamada, T. Gentamicin inhibits Na+-dependent d-glucose transport in rabbit kidney brush-border membrane vesicles. Biochim. Biophys. Acta 858:153–160; 1986.
Hull, R. N.; Cherry, W. R.; Weaver, G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK1. In Vitro 12:670–677; 1976.
Ikeda, T. S.; Hwang, E. S.; Coady, M. J.; Hirayama, B. A.; Hediger, M. A.; Wright, E. M. Characterization of a Na+/glucose cotransporter cloned from rabbit small intestine. J. Membr. Biol. 110:87–95; 1989.
Kanai, Y.; Lee, W.-S.; You, G.; Brown, D.; Hadiger, M. A. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for d-glucose. J. Clin. Invest. 93:397–404; 1994.
Kempson, S. A.; McAteer, J. A.; Al-Mahrouq, H. A.; Dousa, T. P.; Dougherty, G. S.; Evan, A. P. Proximal tubule characteristics of cultured human renal cortex epithelium. J. Lab. Clin. Med. 113:285–296; 1989.
Koepsell, H.; Fritzsch, G.; Korn, K.; Madrala, A. Two substrate sites in the renal Na(+)-d-glucose cotransporter studied by model analysis of phlorizin binding and d-glucose transport measurements. J. Membr. Biol. 114:113–132; 1990.
Koepsell, H.; Spangenberg, J. Function and presumed molecular structure of Na+-d-glucose cotransport systems. J. Membr. Biol. 138:1–11; 1994.
Kong, C.-T.; Yet, S.-F.; Lever, J. E. Cloning and expression of a mammalian Na+/amino acid cotransporter with sequence similarity to Na+/glucose cotransporters. J. Biol. Chem. 268:1509–1512; 1993.
Lambotte, S.; Veyhl, M.; Kohler, M.; Morrison-Shetlar, A. I.; Kinne, R. K.; Schmid, M.; Koepsell, H. The human gene of a protein that modifies Na(+)-d-glucose co-transport. DNA Cell Biol. 15:769–777; 1996.
Lash, L. H. Susceptibility to toxic injury in different nephron cell populations. Toxicol. Lett. 53:97–104; 1990.
Lever, J. E. Regulation of dome formation in kidney epithelial cell cultures. Ann. NY Acad. Sci. 372:371–383; 1981.
Lever, J. E. Expression of a differentiated transport function in apical membrane vesicles isolated from an established kidney epithelial cell line. Sodium electrochemical potential-mediated active sugar transport. J. Biol. Chem. 257:8680–8686; 1982.
Mackenzie, B.; Loo, D. D. F.; Panayotova-Heiermann, M.; Wright, E. M. Biophysical characteristics of the pig kidney Na+/glucose cotransporter SGLT2 reveal a common mechanism for SGLT1 and SGLT2. J. Biol. Chem. 271:32678–32683; 1996.
Mackenzie, B.; Panayotova-Heiermann, M.; Loo, D. D. F.; Lever, J. E.; Wright, E. M. SAAT1 is a low affinity Na+/glucose cotransporter and not an amino acid transporter. A reinterpretation. J. Biol. Chem. 269:22488–22491; 1994.
Misfeldt, D. S.; Sanders, M. J. Transepithelial glucose transport in cell culture. Am. J. Physiol. (Cell Physiol. 9) 240:C92-C95; 1981.
Moran, A.; Handler, J. S.; Turner, R. J. Na+-dependent hexose transport in vesicles from cultured renal epithelial cell line. Am. J. Physiol. (Cell Physiol. 12) 243:C293-C298; 1982.
Moran, A.; Turner, R. J.; Handler, J. S. Regulation of sodium-coupled glucose transport by glucose in a cultured epithelium. J. Biol. Chem. 258:15087–15090; 1983.
Oulianova, N.; Berteloot, A. Sugar transport heterogeneity in the kidney: two independent transporters or different transport modes through an oligomeric protein? 1. Glucose transport studies. J. Membr. Biol. 153:181–194; 1996.
Pajor, A. M.; Hirayama, B. A.; Wright, E. M. Molecular evidence for two renal Na+/glucose transporters. Biochim. Biophys. Acta 1106:216–220; 1992.
Peng, H.; Lever, J. E. Polyamine regulation of Na+/glucose symporter expression in LLC-PK1 cells. J. Cell. Physiol. 154:238–247; 1993.
Poppe, R.; Karbach, U.; Gambaryan, S.; Wiesinger, H.; Lutzenburg, M.; Kraemer, M.; Witte, O. W.; Koepsell, H. Expression of the Na+-d-glucose cotransporter SGLT1 in neurons. J. Neurochem. 69:84–94; 1997.
Quamme, G.; Freeman, H. Evidence for a high-affinity sodium-dependent d-glucose transport system in the kidney. Am. J. Physiol. (Renal Fluid Electrolyte Physiol. 22) 253:F151-F157; 1987.
Rabito, C. A.; Ausiello, D. A. Na+-dependent sugar transport in a cultured epithelial cell line from pig kidney. J. Membr. Biol. 54:31–38; 1980.
Reinhardt, J.; Veyhl, M.; Wagner, K.; Gambaryan, S.; Dekel, C.; Akhoundova, A. Korn, T.; Koepsell, H. Cloning and characterization of the transport modifier RS] from rabbit which was previously assumed to be specific for Na+-d-glucose cotransport. Biochim. Biophys. Acta 1417:131–143; 1999.
Roigaard-Petersen, H.; Jacobsen, C.; Sheikh, M. I. Characteristics of d-galactose, transport systems by luminal membrane vesicles from rabbit kidney. Biochim. Biophys. Acta 856:578–584; 1986.
Ruegg, C. E.; Gandolfi, A. J.; Nagle, R. B.; Brendel, K. Differential patterns of injury to the proximal tubule of renal cortical slices following in vitro exposure to mercuric chloride, potassium dichromate, or hypoxic conditions. Toxicol. Appl. Pharmacol. 90:261–273; 1987.
Ruegg, C. E.; Mandel, L. J. Bulk isolation of renal PCT and PST 1. Glucose-dependent metabolic differences. Am. J. Physiol. (Renal Fluid Electrolyte Physiol. 28) 259:F164-F175 1990.
Sakhrani, L. M.; Badie-Dezfooly, B.; Trizna, W.; Mikhail, N.; Lowe, A. G.; Taub, M.; Fine, L. G. Transport and metabolism of glucose by renal proximal tubular cells in primary culture. Am. J. Physiol. (Renal Fluid Electrolyte Physiol. 15) 246:F757-F764; 1984.
Segal, S.; Rosenhagen, M.; Rea, C. Developmental and other characteristics of alpha-methyl-d-glucoside transport by rat kidney cortex slices. Biochim. Biophys. Acta 291:519–530; 1973.
Silverman, M. The chemical and steric determinants governing sugar interactions with renal tubular membranes. Biochim. Biophys. Acta 332:248–262; 1974.
Taub, M.; Livingston, D. The development of serum-free hormone-supplemented media for primary kidney cultures and their use in examining renal functions. Ann. NY Acad. Sci. 372:406–421; 1981.
Trifillis, A.; Del Valle, P. L.; Cui, X.; Lipsky, M.; Ruegg, C.; Kane, A. S. Cultured rabbit renal proximal straight tubular cells are more susceptible to mercuric chloride than convoluted tubular cells. In Vitro Mol. Toxicol. 11:133–138; 1998.
Turk, E.; Zabel, B.; Mundlos, S.; Dyer, J.; Wright, E. M. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 350:350–356; 1991.
Turner, R. J.; Moran, A. Stoichiometric studies of the renal outer cortical brush border membrane d-glucose transporter. J. Membr. Biol. 67:73–80; 1982a.
Turner, R. J.; Moran, A. Further studies of proximal tubular brush border membrane d-glucose transport heterogeneity. J. Membr. Biol. 70:37–45; 1982b.
Turner, R. J.; Moran, A. Heterogeneity of sodium-dependent d-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am. J. Physiol. (Renal Fluid Electrolyte Physiol. 11) 242:F406-F414; 1982c.
Veyhl, M.; Spangenberg, J.; Puschel, B.; Poppe, R.; Dekel, C.; Fritzsch, G.; Haase, W.; Koepsell, H. Cloning of a membrane-associated protein which modifies activity and properties of the Na(+)-d-glucose cotransporter. J. Biol. Chem. 268:25041–25053; 1993.
Wells, R. G.; Mohandas, T. K.; Hediger, M. A. Localization of the Na+/glucose cotransporter gene SGLT2 to human chromosome 16 close to the centromere. Genomics 17:787–789; 1993.
Yoneyama, Y.; Lever, J. E. Increased trehalase expression after glucose limitation of LLC-PK1 clones. Am. J. Physiol. (Cell Physiol. 24) 255:C816-C821; 1988.
You, G.; Lee, W.-S.; Barros, E. J. G. et al. Molecular characteristics of Na+/coupled glucose transporters in adult and embryonic rat kidney. J. Biol. Chem. 270:29365–29371; 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Valle, P.L., Trifillis, A., Ruegg, C.E. et al. Characterization of glucose transport by cultured rabbit kidney proximal convoluted and proximal straight tubule cells. In Vitro Cell.Dev.Biol.-Animal 38, 218–227 (2002). https://doi.org/10.1290/1071-2690(2002)038<0218:COGTBC>2.0.CO;2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1290/1071-2690(2002)038<0218:COGTBC>2.0.CO;2